Hyperacusis Research sponsored an exciting event at the 2017 ARO Midwinter Research Meeting. The roundtable meeting, which included 41 attendees, focused on defining the key next steps for researching hyperacusis to find a cure. Pictured above: Tanisha Hammill, Rich Salvi, Bryan Pollard
 Notable attendees included:
Notable attendees included:
– Roger Miller, PhD, Division of Scientific Programs, NIH, NIDCD
– Catherine Weisz, PhD, Division of Intramural Research, NIH, NIDCD
– M. Charles Liberman, Professor of Otolaryngology, Harvard Medical School
– Jonathon Whitton, AuD, Senior Audiologist at Decibel Therapeutics
– Larry Roberts, PhD, Department of Psychology, Neuroscience & Behaviour, McMaster University
– Nadine Dehgan, CEO, Hearing Health Foundation
– Torryn Brazel, Executive Director & COO, American Tinnitus Association
– Tanisha Hammill, Senior Research Administrator, DOD, Hearing Center of Excellence
Bryan Pollard, President of Hyperacusis Research, kicked off the evening, highlighting the progress made to date for the cause of hyperacusis.
– Media attention continues to grow with new articles this year in Tinnitus Today, Hearing Health, USA Today, and Classical Singer describing the challenges of hyperacusis.
– Almost $100,000 has been raised for hyperacusis research in the past 2 years.
– In the past 4 years, the number of research papers in PUBMED on hyperacusis has more than doubled.
– In the last 5 years, the NIH funding for hyperacusis projects has more than doubled, with more than $1.8 Million in funding in 2016.
 Joyce C. was our “ask the patient” representative. She noted that she was one of the few patients well enough to travel to ARO and attend our dinner discussion, and only with plenty of hearing protection.
Joyce C. was our “ask the patient” representative. She noted that she was one of the few patients well enough to travel to ARO and attend our dinner discussion, and only with plenty of hearing protection.
Her severe injury came from workplace noise nearly 10 years ago. Since then, she has spent most of her time in a quiet home, always protecting her ears when in an uncontrolled noise setting. Over the years, she has improved a great deal. “I am out of pain, though I know I am one loud noise away from catastrophe.”
Joyce mentioned the huge disconnect between patients and “experts” — doctors, audiologists and clinical researchers — with “expert” information being far from the reality experienced by most patients. This disconnect translates into medical advice that isn’t just wrong in the abstract but that causes great harm to patients.
She compared noise-induced pain with Superior Canal Dehiscence Syndrome (SCDS), which was identified in the 1990s as a new disorder in the field of otology. “Before that, people who heard their eyeballs move were crazy,” she said. “After that, they had a hole in their temporal bone. Some 20-odd years later, hyperacusis with pain is following that path, as the latest disorder in the field.”
She also noted that the pain from hyperacusis lies on a continuum from mild to suicidal, with no limit on how bad it can become, and often presents as just one of a constellation of difficult auditory symptoms
Researchers wrote their questions on index cards. A sampling:
Q. What would help you in daily life?
A. A volume limiter on the world. Failing that, ear protection strong enough to block out sirens and construction noise — in other words, strong enough to protect sufficiently against the worst noises someone would encounter.
Q. Is there any pitch difference in the hyperacusis experience? For example, a low pitch is OK but you hate high pitches?
A. Yes. High pitches and low throbs are the worst. Frequency is a huge component.
Q. Does your hyperacusis have good and bad days? If yes, what influences it?
A. Not in the short term. It tends not to fluctuate on a day-to-day basis, though it worsens with noise exposure. The only thing that influences it is noise. Improvement is measured in months, with time and silence, whereas worsening happens in an instant with noise.
Q. What activity do you most want to return to? Allow me to rephrase: What is the activity that would be most meaningful to you if you were able to re-engage?
A. I long to communicate normally.
The agenda for the main discussion of the event followed our Roadmap to a Cure.
I. Path A: Identify Hyperacusis Mechanisms
 Bryan Pollard gave a preliminary review of our comprehensive Sanford CoRDS hyperacusis survey. This preview was based on the first 100 participants (many more have now completed the survey). Some interesting insights from this survey are listed below:
Bryan Pollard gave a preliminary review of our comprehensive Sanford CoRDS hyperacusis survey. This preview was based on the first 100 participants (many more have now completed the survey). Some interesting insights from this survey are listed below:
– There is a fairly even split between those whose hyperacusis started suddenly versus gradually.
– 90% also have tinnitus.
– 66% experience daily or continuous pain from everyday noises.
– The majority experience new onsets of ear pain when exposed to a new loud sound.
– For almost all participants, the new onset of pain is immediate upon a new noise exposure.
– For 28% the new onset of pain lasts several days and for 25% it lasts for 5 to 24 hours.
– There is a broad distribution of pain types: sharp, dull, stabbing, burning and throbbing.
– To recover from a new setback, it takes several days for 41% and several weeks for 27%.
Researchers are starting to acquire this survey dataset to do a more rigorous analysis and we expect this to yield important new insights – particularly pertaining to those who experience hyperacusis with pain.
Understanding mechanical mechanisms related to hyperacusis: Heidi Nakajima, Assistant Professor of Otolaryngology at Harvard Medical School and Xiying Guan, Postdoctoral Research Fellow at the Dept. of Otolaryngology, Harvard Medical School and recipient of our 2016 grant award.
Xiying summarized his work on conductive hyperacusis which is caused by passive mechanical changes. This subtype of hyperacusis is increasingly reported in literature because clinicians have recently recognized that hyperacusis is a common consequence of Superior Canal Dehiscence (SCD), an abnormal opening in the inner ear. Symptoms of conductive hyperacusis in SCD patients include being able to hear their eyeballs move; their own voice becomes very loud and distorted; they hear footsteps, neck movement, and eating or chewing sounds. Their hearing is hypersensitive to machine generated vibrations.
Hyperacusis is common among SCD. It is reported in histological and radiological studies on human temporal bones that the prevalence rate of SCD is 0.5% to 1.9%. Xiying further explained that by the smaller number, 0.5%, about 1.5 million people have SCD in the US. In a survey examining 62 ears with CT-confirmed SCD, hyperacusis occurred in 30% of the ears. Therefore, about a half million people in the US have SCD-associated hyperacusis.
To investigate the mechanism of conductive hyperacusis, Xiying is using a novel intracochlear pressure measurement by using tiny fiberoptic sensors in human cadavers to measure the sound pressures in the inner ear, and thus estimate the hearing. The background of this work can be found in this 2008 paper “Differential Intracochlear Sound Pressure Measurements in Normal Human Temporal Bones.”
Below is a schematic of a human ear. On the right is the vestibular system with the seimicircular canals, responsible for balance and motion. On the left is the cochlea, the hearing organ. Sound enters the ear canal and causes vibrations of the middle ear bones. Movement of the stapes footplate displaces the fluid in the cochlea and generates pressure in two fluid channels- scala vestibuli (SV) and scala tympani (ST).
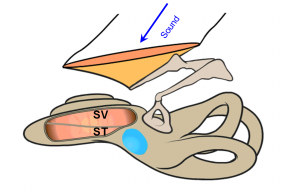 The round window sitting at the scala tympani (blue oval area in diagram), acts as a pressure relief. Because of that, the pressure in the scala tympani is lower than that in the vestibuli at the base of the cochlea. Thus there is a pressure difference across the basilar membrane. The pressure difference generates the traveling wave on the basilar membrane, which stimulates the hair cells and results in hearing. Thus, the differential pressure is the cochlear input drive with the formula: PSV− PST = ΔP (Cochlear Input Drive).
The round window sitting at the scala tympani (blue oval area in diagram), acts as a pressure relief. Because of that, the pressure in the scala tympani is lower than that in the vestibuli at the base of the cochlea. Thus there is a pressure difference across the basilar membrane. The pressure difference generates the traveling wave on the basilar membrane, which stimulates the hair cells and results in hearing. Thus, the differential pressure is the cochlear input drive with the formula: PSV− PST = ΔP (Cochlear Input Drive).
 It was shown in animals that the Cochlear drive (ΔP) estimates hearing as seen the graph, where the Cochlear Microphonic in general is aligned to Cochlear drive in both magnitude and phase over the frequencies. Since in a human cadaveric ear the neurophysiological response cannot be measured, this tool will enable hearing to be estimated.. Graph from Dancer and Franke, Hearing Research 1980 Guinea Pig and Lynch, Nedzelnitsky and Peake, JASA 1982 Cat.
It was shown in animals that the Cochlear drive (ΔP) estimates hearing as seen the graph, where the Cochlear Microphonic in general is aligned to Cochlear drive in both magnitude and phase over the frequencies. Since in a human cadaveric ear the neurophysiological response cannot be measured, this tool will enable hearing to be estimated.. Graph from Dancer and Franke, Hearing Research 1980 Guinea Pig and Lynch, Nedzelnitsky and Peake, JASA 1982 Cat.
Xiying went on to describe how he has used this technique to demonstrate the effect of SCD on hearing and balance and the potential impact of surgical interventions to alleviate hyperacusis and other SCD symptoms. We are especially interested in how concepts related to this measurement technique could be utilized to explore and elucidate the underlying mechanism(s) of mechanical pathologies causing hyperacusis. Then we can develop and determine effective treatments for the identified conditions
II. Path B: Elucidation of Hyperacusis Mechanisms
Auditory Nociception, Type II Afferents, and Pain Hyperacusis: Jaime García-Añoveros, Department of Anesthesiology, Neurology and Physiology, Northwestern University.
Jaime provided a brief background and key questions that need investigation related to pain mechanisms with hyperacusis. Auditory nociception is defined as the detection of cochlear damage (such as that caused by intense or persistent noise) by Type II afferents and its communication to the brain (cochlear nucleus). Jaime reviewed these questions:
1) What genes and proteins are used by Type II afferents?
2) What brain areas other than the cochlear nucleus participate in auditory nociception?
3) Does auditory nociception/Type II afferents mediate or contribute to pain hyperacusis?
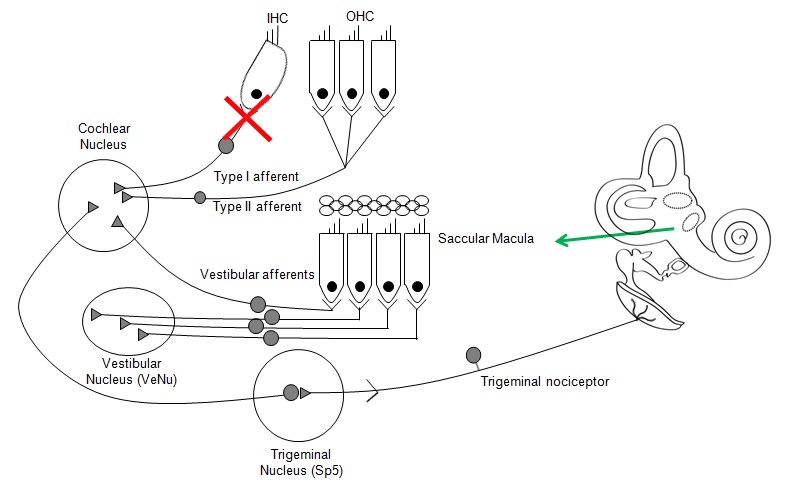
Jaime presented work that helps to demonstrate the potential paths of communication to the brain following cochlear damage. In the diagram below, Jaime described how key pathways of communication were ruled out. He showed work in animal models where various segments of the auditory pathway were segmented and cochlear nucleus activity was monitored. By systematically ruling out Type I afferents, Vestibular afferents, and Trigeminal neural activity, the function of the Type II afferents was established.
Jaime described how certain processes such as protective reflexes and neurogenic inflammation could lead to noise-induced pain sensations. Future work is needed to investigate the minimal damage needed to set up this pain pathway and how activation of the pain process could be initiated.
Type II Fibers and Pain: Paul Fuchs, Bordley Professor of Otolaryngology-Head and Neck Surgery at Johns Hopkins School of Medicine, provided more detail on the Type II fibers.
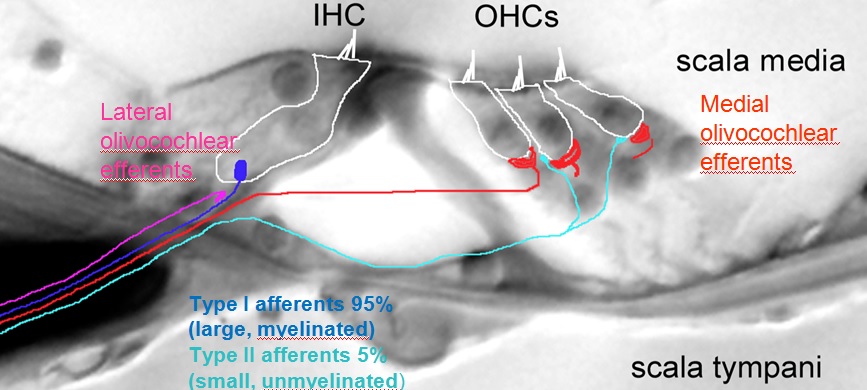
Paul reviewed his work, which has demonstrated the differences of cochlear nerve fibers. The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it – 95% of all afferents. Type II afferents branch extensively to contact numerous outer hair cells for functions largely unknown. Medial efferent neurons synapse on outer hair cells and when active, they inhibit the cochlea. Lateral efferent neurons synapse on Type I afferents beneath inner hair cells with functions largely unknown. See image below for the layout of Type I and Type II nerve fibers in the cochlea.
Type II afferents are poorly activated by hair cell glutamate release, but respond strongly to hair cell damage (C. Weisz, C. Liu) Ex vivo recordings from type II afferents are carried out in cochlear coils excised from young rats or mice. During the recording, a visible marker fills the neuron, revealing the characteristically extensive dendrite that extends hundreds of microns toward the cochlear base (higher frequencies). Each type II afferent makes synaptic connections with ~20 outer hair cells (see image to the right).
Paul explained that future progress will be accelerated using animal models in which ‘biomarker’ genes can be used to modulate type II afferent activity. This will enable a better understanding of circumstances that lead to their activation and uncover potential relationships to hyperacusis.
Sensitive Method for Assessing Noise and Drug Induced Hyperacusis in an Animal Model: Kelly Radziwon, University at Buffalo Center for Hearing and Deafness, recipient of our 2015 grant award.
 Kelly described the background of her work using auditory reaction time (RT) as a measure of loudness recruitment and hyperacusis. Besides elevating hearing threshold, sensorineural hearing loss dramatically alters loudness growth, often leading to loudness recruitment. However, in some cases, individuals develop hyperacusis, a condition in which suprathreshold sounds are perceived as much louder than normal at moderate to high intensities. To assess animals for noise-induced loudness hyperacusis, Kelly obtained behavioral loudness growth functions by measuring reaction time-intensity (RT-I) functions before and after inducing a high-frequency hearing loss.
Kelly described the background of her work using auditory reaction time (RT) as a measure of loudness recruitment and hyperacusis. Besides elevating hearing threshold, sensorineural hearing loss dramatically alters loudness growth, often leading to loudness recruitment. However, in some cases, individuals develop hyperacusis, a condition in which suprathreshold sounds are perceived as much louder than normal at moderate to high intensities. To assess animals for noise-induced loudness hyperacusis, Kelly obtained behavioral loudness growth functions by measuring reaction time-intensity (RT-I) functions before and after inducing a high-frequency hearing loss.
In terms of loudness growth, loudness recruitment is defined as a steeper than normal growth of loudness at intensities just above threshold, with normal loudness growth at high sound intensities. In contrast, hyperacusis is characterized by a steeper growth of loudness at both moderate and high sound intensities, resulting in moderate to high-intensity sounds being perceived as louder than normal. Therefore, using a RT-I paradigm, individuals with hyperacusis have faster-than-normal reaction times at moderate to high sound intensities whereas those with loudness recruitment have normal reaction times at moderate and high sound intensities.
Kelly’s animal subjects were continuously exposed to a narrowband noise (16-20 kHz) at 102 dB SPL for 7 weeks. Behavioral RT-I functions to tone bursts and audiograms were measured pre- and 2 months post-exposure. Kelly found that prolonged exposure to high-frequency noise induced behavioral evidence of loudness recruitment in the region of high-frequency hearing loss (see 20 and 26 kHz graphs below)) while producing hyperacusis-like behavior in some animals in the low-frequency regions (see 4kHz graph below) where hearing thresholds were normal. Future studies are planned to determine the underlying neural and biochemical correlates in animals that develop noise-induced loudness hyperacusis.
Animal Models of Avoidance and Pain Hyperacusis: Senthilvelan Manohar, University at Buffalo Center for Hearing and Deafness
Senthil’s work has focused on the development of two types of hyperacusis animal models: pain and avoidance. This is a critical next step in the roadmap for pain hyperacusis. Senthil described this test as an Auditory Nociception Test (ANT). In the field of pain research, it has been well established that the timing for mice to remove their tail from heat is correlated to pain thresholds (http://jpet.aspetjournals.org/content/72/1/74). It is proposed with ANT that the latency to remove the tail from warm water can be an indicator of the level of nociception.
In his work, Senthil is testing different loudness levels. A longer latency will indicate hypoalgesia (decreased sensitivity to painful stimuli) while a shorter latency will indicate hyperalgesia (increased sensitivity to pain).
Additional work is being done with an avoidance model. Hyperacusis Research is excited to see the development of an auditory nociception test, which will be critical for uncovering the mechanisms of pain hyperacusis.
III. Path C: Development and Optimization of Therapy
Improving options for Hyperacusis Patients: Tom Lobl and Freddie Daver, The Alfred E. Mann Institute for Biomedical Engineering at the University of Southern California.
Freddie Daver provided an overview of the Alfred Mann Institute (AMI). The mission of AMI is to accelerate commercialization of medical device technology with goal of improving human health and well being. AMI is a 501c3 non-profit that includes a cross disciplined team organized for transformational and translational technology focusing on commercial development.
Freddie described AMI’s interest in helping to develop a product that could benefit hyperacusis sufferers. To pursue this investigation, AMI is analyzing the Sanford CoRDS survey data to identify common themes and needs. Hyperacusis Research is excited for AMI’s interest in hyperacusis. If you have ideas of a product for hyperacusis sufferers please let us know and we will pass the idea along to Tom and Freddie.
IV. Path D: Determine Hyperacusis Cure
Identifying the Neural Network(s) for Hyperacusis: Rich Salvi, Director Center for Hearing & Deafness University at Buffalo, Scientific Advisor to Hyperacusis Research
Rich started by describing the most common brain imaging techniques to identify neural networks – Positron Emission Tomography (PET)-slow, Magnetic Resonance Imaging (MRI)-moderate speed, Magnetoencephalography (MEG)-fast, and Quantitative Electroencephalography (qEEG)-fast. MRI is very popular due to the detail that can be obtained but MRI machines are very loud and not suitable for many with hyperacusis.
Rich showed an example of how fMRI can be used to show correlation between brain regions. For example, the Left Motor Cortex BOLD (Blood-Oxygen-Level Dependent contrast imaging signal) is highly correlated to the Right Motor Cortex. However the Left Motor Cortex is only weakly correlated to the Visual Cortex.
Sodium Salicylate (SS) alters functional connectivity in specific brain regions. Rich explained the image below, which has ROI functional connectivity heat maps showing the regions of the brain where SS induced a statistically significant increase in functional connectivity with the ROI placed in the ACx (top row), MGB (middle row) or IC (bottom row).
Brain Region Labels: CB4, lobules 4 of cerebellum; PFL, parafloccular lobe of cerebellum; RN, gigantocellular reticular nucleus, IC, inferior colliculius; MGB, medial geniculate body; ACx, auditory cortex; HIP, hippocampus; AMY, amygdala.
As can be seen, there are numerous brain regions showing increased connectivity with tinnitus/hyperacusis. Each of these areas offer potential insights into the neural underpinnings of hyperacusis, which can help uncover the mechanisms and lead to better treatment options.
From Basic Discovery Science through Clinical Research: Deborah Hall, Nottingham Hearing Biomedical Research Unit, University of Nottingham
Deborah described the process for prioritizing a research agenda with a pathway that spans basic discovery science through to clinical research and readiness for respective research activities with learning from European models for a tinnitus research strategy.
Deborah’s first emphasis was on reducing research waste. An efficient system of research should address health problems of importance to populations and the interventions and outcomes considered important by patients and clinicians. Problems range from choosing the wrong questions for research to doing studies that are unnecessary or poorly designed. Some of these can be mitigated by increasing the involvement of patients and clinicians in shaping research agendas and specific questions for research. See Research Waste details from Chalmers & Glasziou Lancet 2009: 374:86-89
http://www.thelancet.com/series/research: January 2014. Given the very limited resources currently available to fund hyperacusis research projects, we are especially interesting in helping to reduce research waste.
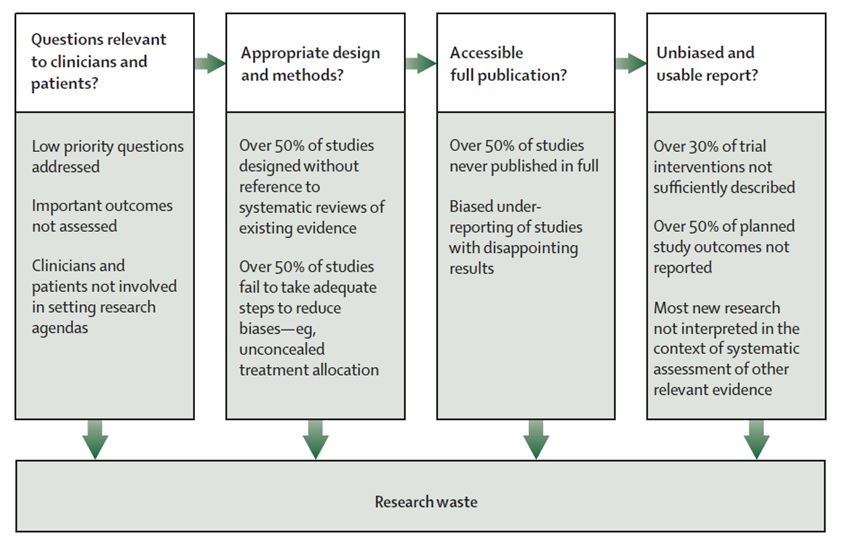
To address these concerns for tinnitus in the UK, the James Lind Alliance was formed. The alliance aims to raise awareness among those who fund health research about what matters to both patients and clinicians so that clinical research is relevant and beneficial to end users. It provides a systematic process driven by patients, family members and clinical professionals. It makes research recommendations that are publicly accessible and produces a top 10 of the most important unanswered research questions.
Deborah concluded with these four key messages:
1) Identify what is known and what are the gaps
2) Develop a research strategy
3) Each strategic question needs a research plan
4) Many components needed to be ready for clinical trial
Hyperacusis Alliance
 Bryan concluded the evening by proposing the formation of a new Hyperacusis Alliance. The mission of the alliance is to provide a platform for communication and coordinated action for those working to increase awareness, funding, and research for hyperacusis. Participation will include:
Bryan concluded the evening by proposing the formation of a new Hyperacusis Alliance. The mission of the alliance is to provide a platform for communication and coordinated action for those working to increase awareness, funding, and research for hyperacusis. Participation will include:
Contributing to hyperacusis information exchange.
Participation in conference calls (~ 4 per year) with an agenda that will rotate with 3 focus areas:
1) Awareness
2) Funding (potential for creating larger multi-agency grant)
3) Research (models & mechanisms , assessment tools, collaboration opportunities).
For further insights into the technical aspects of the discussions at ARO, view Dr. Iver Juster’s Observations from the 2017 ARO Midwinter Meeting.
Additional Event Pictures
















Hi,
This was very helpful as I also suffer from Hyperacusis. Is there support groups available?
Sorry we do not have support groups at this time although the American Tinnitus Association has support groups which include hyperacusis.
I am 56 years old and I have had hyperacusis since childhood. I don’t know if this has anything to do with it, but playing as a child,I jumped off a bunk bed onto a lower bed and crashed my head onto a cast-iron radiator. I have to run from breathing and snoring sounds, and have discomfort from drumbeat sounds and other music sounds, especially coming through walls. I lived in fear all my life of needing to go to a hospital because I couldn’t afford my own room.Family travel to hotels meant I had to somehow sleep on the bathroom floors. And now, although maybe premature, I’m afraid of being in a nursing home with a snoring,old woman next to me. And topping this all off, I have suffered most of my life with mental illness(Bipolar Disorder. I would really like to have a device that would go on my ear and make a loud noise blocking out these sounds. Special ear molds do nothing to keep me from hearing these sounds.
I also suffer from Hyperacusis very badly. Please help us find a cure.
I would be glad to have such a device too. I am totally blind with no light perception due to ROP stage 5 from birth on, and I have hyperacusis from babyhood. I never stood loud noises, always got afraid from them. I was never examined, doctors don’t care about it, they say, I lost my mind, it cannot be ttrue, it is just because my totally blindness, that’s all. They say sounds aren’t loud just I imagine it. I really don’t know what to do in this case, I am from Hungary, a little not developed country.
I am 28. I suffer from hyperacusis since 22. Hyperacusis took my all life. I am not different then a dead. And i am gonna be honest i do not hope any cure in the future. U all doctors spend hyperacusis charity monies to hotels, foods and your alcohols for meetings with some short bullshit speech. Thank is the reality.
I also suffer from hyperacusis it started when I worked in a nursing home a patient there who whistled continuously at a very high pitch caused mine over a period of one year. I couldn’t stand it anymore I had to quit my job. I went to an ent they diagnosed me & said he couldn’t do anything for me & sent me home crying. No body understands how painful this is. I went to my physician she didn’t see anything wrong & wouldn’t give me anything for the pain. Is there any doctors out there that will prescribe pain medicine for this? I live in Des Moines Iowa.
I have ht with pain, hard pain more then electricity of 220v, also shock for 2 Seconds and intense pain close after the noise has already disappeared during the market, what can be done, all the time in the street with noise-reducing headphones plus music from the tinnitus mask and the intensity of the noise outside. You may be informed where you can perform the solitude for neural or cerebral damage, for example Meg. There is no ability to work as ever if at all, the cause of injury and noise trauma.Minor balance disorders and blurring of vision, with spatial understanding, are also present due to hyperkisopathy and neurological hearing impairment to easy, bilateral. Can anyone researchers give contact email?
Sorry, I ment to say that I have n s hearing loss 2 years after the hyperacusisabt tinnitus happened.