An important function of Hyperacusis Research is to accelerate research by connecting researchers to the challenges of patients’ hyperacusis condition including noise-induced pain. We meet with researchers regularly to discuss the planned research and probe to see how to investigate the complex realities of patients’ experiences. For example, experiments are often looking for responses from one primary noise exposure while patients may experience a more complicated situation of moderate noise exposures after the primary event. We explore ways experiments can simulate these real life conditions.
In 2015, Bryan Pollard, President of Hyperacusis Research, visited Rich Salvi and his team at the Center for Hearing and Deafness at SUNY Buffalo. Dr. Salvi, who is a Scientific Advisor for Hyperacusis Research, also gave our first online Webinar. Professor Salvi indicated that Hyperacusis Research has played an important role in helping to focus the research efforts at SUNY on hyperacusis with pain. We are extremely grateful for their efforts to continue to aggressively pursue a cure for hyperacusis.
Kelly Radziwon, a post-doctoral fellow, described her research including the current work related to the grant she received from Hyperacusis Research via our partner the Hearing Health Foundation. This research will investigate the relationship between pain-associated proteins in the auditory pathway and hyperacusis.
Despite the apparent link between pain and hyperacusis in humans, little research has been conducted directly comparing the presence of inflammation along the auditory pathway and the occurrence of hyperacusis. Kelly will be using reaction time measurements to assess rats for hyperacusis. Following noise exposure, rats will be deemed to have hyperacusis if they have faster-than-normal reaction times to moderate and high-level sounds. The goal is to correlate the presence of pain-related molecules along the auditory pathway with reliable behavioral measures of drug and noise-induced hyperacusis. Additionally, Kelly hopes to determine the biological correlates of this hyperacusis. Given that ear pain often co-occurs with hyperacusis, Kelly will search for the expression of pain-related molecules in the auditory system.
This will help determine the relationship between pain-related molecules and inflammation along the auditory pathway and the perceptual experience of hyperacusis. Kelly will initially focus on three pain related molecules: Substance P (SP), Neurokinin 1 (NK1), and transient receptor potential cation channel subfamily V member 1 (TRPV1). Substance P is an important element in pain perception. The sensory function of substance P is related to the transmission of pain information into the central nervous system. The endogenous receptor for substance P is receptor NK1. TRPV1 is a commonly studied pain receptor since it is activated by a capsaicin (the chemical responsible for chili peppers tasting hot). Activation of TRPV1 receptors on peripheral neurons produces a burning sensation. If these molecules are correlated with hyperacusis, this will provide the biological markers needed for the development of treatments for ear pain and hyperacusis.
In a recent update of her work Kelly stated that she has been using the behavioral reaction time paradigm to measure noise-induced hyperacusis in rats. Importantly, Kelly has been correlating these reaction time measures with the hearing loss profile in each animal before, during, and after the noise trauma. In addition to behavioral experiments, Kelly has also been using Western blotting to quantify the expression of the pain-related protein, substance P, in both the cochlear nucleus and inferior colliculus of control and noise-exposed rats.
A key enabling aspect of Kelly Radziwon’s work has been evaluating methodologies used for animal testing for tinnitus and hyperacusis. At the 2015 TRI Conference, Kelly presented work showing results from a behavioral paradigm that can reliably distinguish hyperacusis, loudness recruitment, and normal loudness growth in rats using reaction time (RT) measures following both sodium salicylate (SS) treatment and noise overexposure. In addition, local field potentials (LFPs) from the auditory cortex (AC) were recorded from another group of rats following both SS injection and an intense unilateral noise exposure. Kelly’s team found that both SS treatment and noise exposure caused hyperacusis-like behavior in a subset of animals and, similarly, resulted in increased cortical responsiveness to sound.
Another researcher at SUNY, Senthilvelan Manohar, is also pursuing research to determine how chronic noise stimulus modulates pain receptors in spiral ganglion neurons and the cochlear nucleus. He is starting by characterizing pain receptors in auditory neurons to see which auditory neurons are expressing these receptors. If spiral ganglion neurons express opioid receptors (such as DOR, KOR and MOR), Senthilvelan will work to determine its role in auditory signaling by two experiments: Experiment 1 – Determine whether chronic moderated and high noise exposure desensitizes or sensitizes these opioid receptors. Experiment 2 – Determine whether desensitizing spiral ganglion neurons opioid receptors by administrating endogenous opioid would increase auditory sensitivity or decrease it. We will keep a close watch on these important research efforts.
Bryan Pollard was also able to meet with Sarah Hayes, a PhD Neuroscience and AuD student, and Dr. Christina Stocking who is a Clinical Associate Professor at the University at Buffalo Speech, Language and Hearing Clinic. Hyperacusis Research has referred a number of hyperacusis patients to the clinic. It is insightful to learn about the clinical experiences with hyperacusis patients. In this discussion, Bryan raised some questions about whether there may be an immunological response occurring within the cochlea of hyperacusis patients. Sarah referred Bryan to Bohua Hu, Associate Professor in the Center for Hearing and Deafness at SUNY.
Bohua Hu has focused his research on the biological and molecular mechanisms of noise-induced hearing loss. In the past few years, Dr. Hu has investigated the cochlear immune response to acoustic injury. These studies revealed active immune reaction after acoustic injury. The noise-induced immune response involves both professional immune cells and cochlear resident cells. Importantly, Hu’s studies showed the activation of adaptive immune reaction after acoustic injury. Because the adaptive immune response can exert a long-term effect on cochlear homeostasis, a deviant immune response can contribute to hyperacusis. In other neuroscience disciplines, the link between immune dysfunction and chronic pain has been established. This finding suggests important roles for chronic inflammation in neurological disorders, such as hyperacusis and tinnitus. While Dr. Hu has not performed specific experiments to study hyperacusis, he is interested in the potential contribution of the immune response to hyperacusis. Dr. Hu discussed a possible research project to investigate the role of immune/inflammation response in the generation and treatment of hyperacusis. He believes that this exploration will provide new insides for a better understanding of biological mechanisms of hyperacusis.
Bryan also asked Dr. Hu the following questions to gain additional insights into this path of research:
1. Are these mechanisms active only when acoustic trauma is significant enough to cause hair cell death (e.g. with noise of 120dB for 1 hour or 100dB for 4 hours)?
Dr. Hu: It is likely that a prolonged, low-level noise exposure would preferentially provoke the response of cochlear tissue macrophages. This response pattern is different from that seen in cochleae sustained from exposure to a high level noise, which causes the infiltration of circulating monocytes. This speculation is supported by our recent study showing the change in cochlear tissue macrophages with the increase of age. A mild or moderate stress (like aging sensory cell degeneration and a low level noise exposure) is likely to affect the tissue macrophages.
2. Could the initial health (immunologically based or inflammatory related issues) of a subject change the amount of immune response with the same acoustic trauma?
Dr. Hu: I think so. Local effects, such as a previous history of noise exposure, medication and aging, could exert a greater impact on the immune response in the cochlea than does a systemic effect (eg. general health).
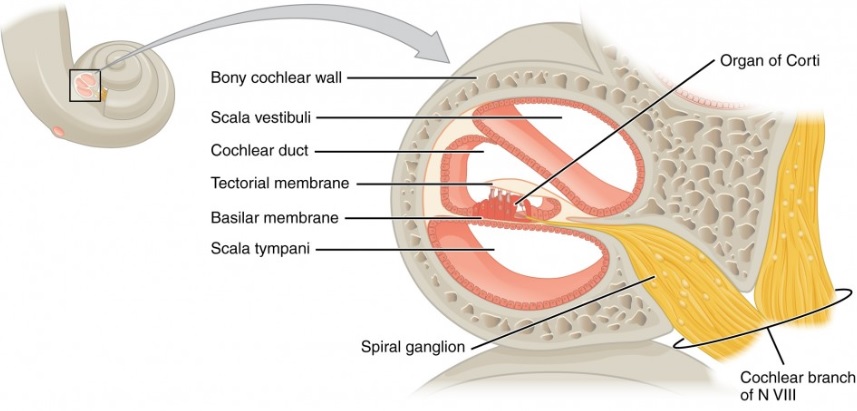
Cross section of the cochlea with sections to investigate for immune response.
3. Once acoustic trauma has occurred, can this immune response be re-initiated by a lower acoustic trauma level?
Dr Hu: We recently published a paper showing the activation of antigen presenting function (Activation of the antigen presentation function of mononuclear phagocyte populations associated with the basilar membrane of the cochlea after acoustic overstimulation). Antigen presentation can lead to activation of the adaptive immune response and can generate a long-term effect on the cochlear immune system. It is likely that the ear can remember a previous acoustic trauma. In terms of how a previous acoustic injury affects the subsequent cochlear immune response, I think that both enhancement and suppression are possible.