This article by Iver Juster MD is the first of a series focusing on hyperacusis insights from the 2022 Association for Research in Otolaryngology (ARO)—and part of a larger Hyperacusis Research series on hyperacusis-related research about the biology, diagnosis, patient experience and treatment of this life-changing condition. In doing so, we’ll sometimes look at research itself: how questions are formulated and answered, and—importantly—how the findings might translate into the real world.
Today, we focus on the big picture of hyperacusis research and a peek at some questions that—directly or indirectly—touch on hyperacusis.
Iver is a member of the Hyperacusis Research Science Advisory Board. He practiced as a primary care physician in California and Oregon and became interested in how physicians and patients gather and use information to make decisions about health. His interest in hyperacusis was kindled when a close family member’s world radically changed after a moderate sound exposure 15 years ago. He says, “Since then, I’ve applied my abilities to curiously connect evolving scientific evidence, research methods, clinicians, patients and researchers to develop an understanding of the experience and biology of hyperacusis.”
Iver has written about hyperacusis conferences here and here.
The ARO’s mission is to encourage and promote basic and clinical research in otolaryngology, hearing and balance, and related fields. ARO has a big international meeting every winter, attended by researchers, clinicians, students, teachers, and businesses involved in the diagnosis and treatment of hearing disorders. (www.aro.org).
This year’s conference touched on a wide range of topics or perspectives important to understanding—and potentially treating—hyperacusis. Understanding hyperacusis will greatly benefit from research in genetics, metabolism, immunology, pharmacology, neuroscience, anatomy, and how all of the above influence perception and its interpretation. Everything in science is in some way connected, so you might think of these categories as faces of the same mountain. This series will include research that isn’t specifically about hyperacusis but may well shed light on what it is and how people with the condition might benefit.
As always, this year’s ARO not only presented fascinating research directly or potentially relevant to hyperacusis, but also both illuminated and raised questions about the extent to which basic science research methods do or can illuminate the biology and the varied expressions of the human condition.
In these articles, I’ll use this classification of research perspectives—faces of the mountain:
| Research perspective | Description |
| Anatomic | Damage to or dysfunction of the structure of components of the auditory system from a variety of causes |
| Inflammatory and immune | Alterations to the structure or function of components of the auditory system due to inflammation or a normal or abnormal immune response |
| Genetic | Hereditary or acquired (such as epigenetic) variations in genes or gene function, resulting in disruption of healthy functioning of the auditory system |
| Epigenetic | Non-genetic changes to DNA that control whether specific genes are turned on or off. When a gene is turned on, it can tell cellular machinery to make its target protein. There are also non-epigenetic factors such as vitamins, minerals, and other small molecules that can turn up or down the actions of genes or their targets in the body. |
| Models (cellular or animal) | Ways to perturb the normal structure or function in a cell, tissue, or animal to elicit behavior that appears to be (or be related to) hyperacusis |
| Neuroscience (including synaptopathy) | Anatomic or functional disruptions of any component of the auditory nervous system from sensation of sound (afferent) through higher-level processing, and feedback (efferent) to lower-level nerves (e.g. back to the afferents) |
| Diagnosis | Distinguishing conditions with sound sensitivity in a way that is useful to researchers, clinicians, and patients. Effective diagnosis is essential for developing, testing, and deploying treatments |
| Treatment | Actions, drugs, and devices that prevent hyperacusis (or setbacks), that cure or improve it, or modify its impact on the patient |
Let’s start with a trip through the auditory pathway—how sounds are perceived, interpreted, and responded to.
(click the image for a larger view)
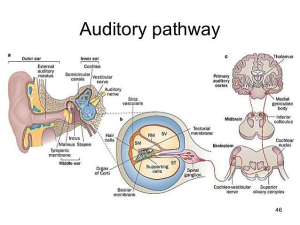
https://image.slidesharecdn.com/theear-150824235451-lva1-app6892/95/the-ear-auditory-pathway-and-olfactory-pathway-46-638.jpg?cb=1440460587. Labels in orange boxes added. ST: Scala tympani; SV: Scala vestibuli; SM: Scala media. RM: Reisner’s membrane.
Key to the auditory pathway diagram: The “sensory apparatus” of hearing consists of the outer, middle, and inner ears. Left picture: The outer ear conducts sound (as pulses of air pressure) to the tympanic membrane (ear drum), which vibrates, transmitting the vibrations to the inner ear via the three linked bones (malleus, incus, and stapes) of the middle ear. The stapes (aka “stirrup bone”) touches a membrane called the round window, which is part of the cochlea. The vibrations of the round window cause the fluid inside the cochlea to vibrate.
Now we’re in the inner ear (middle picture shows the cochlea in cross-section). The cochlea is coiled and filled with fluid in its three compartments (scalae). The vibrations in the fluid cause the basilar membrane to vibrate, which activates specialized inner hair cells (IHCs) depending on the frequency of the sound (high frequencies closer to the basal turn of the cochlea, and low frequencies closer to the apex of the cochlea). When IHCs are activated, they transmit that information to a nerve ending (synapse) that touches the base of the cell, transmitting electrical impulses upwards to the brain (left picture). The bodies of the nerves that touch IHCs are in the spiral ganglion. Nerves coming from the IHCs are called Type I auditory neurons.
In the cochlea are one row of IHCs (dedicated to hearing) and three rows of outer hair cells (OHCs) which also send signals up the line under certain conditions. Their life doesn’t seem to be as tightly organized (or maybe not as well understood) as IHCs. They are involved in controlling the intensity of information that flows up to the brain (and our perception)—turning it up when very soft, but also, they seem to respond to very loud sounds, possibly to tell the brain to turn down the incoming signal from the IHCs for self-protection. The synapses touching the OHCs generate electrical impulses along Type II auditory neurons and their cell bodies reside in the spiral ganglion. About 95% of nerve cell bodies in the spiral ganglion are from Type I neurons and about 5% are from Type II. The spiral ganglion nerves synapse in the cochlear nuclei, as shown in the picture above.
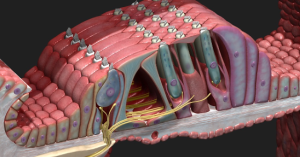
Here is a picture from https://3d4medical.com/blog/cochlea to help visualize the inner (to the left) and outer (to the right) hair cells with Types I and II auditory neurons synapsing on them and carrying information higher up in the central nervous system.
Here is a recent article on Types I and II neurons: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5393967/. Though the narrative is rather scientific, it’s a good review of the state of our understanding as of a few years ago and has excellent illustrations.
It appears that the Type II neurons are responsible for some of the pain sensation in hyperacusis (not all patients with hyperacusis have pain). However, the origin of pain in hyperacusis is probably complex; for example, in some people there appears to be a partial or major contribution from a reflex tightening of the very tiny tensor tympani muscle, which pulls on one of the three middle ear bones to dampen loud sounds. You can see a nice picture of the tensor tympani here: https://www.scienceabc.com/humans/tensor-tympani-muscle-why-does-closing-the-eyes-tightly-produce-a-rumbling-sound-in-the-ears.html.
It may be that more-or-less chronic tightening of the tensor tympani (in an effort to protect the eardrum from vibrating too severely) is connected to tightening of other muscles in the throat and jaw. Some people with hyperacusis report that pain and tightening of their jaw muscles (including those inside the jaw – you can feel them (carefully!) by hooking your little finger and reaching inside your mouth way back in your cheeks) is part of their reaction to sound.
What happens higher up in the central nervous system is more of a mystery, but it seems that this “central sensitization” is a big part of the picture for people with hyperacusis (whether or not pain is involved). Central sensitization occurs in many conditions that involve chronic pain; probably it developed as a protective mechanism but in hyperacusis is way too sensitive. A much deeper understanding of central sensitization will help improve our ability to treat (and possibly prevent) not only hyperacusis but many other neurological conditions.
The closer we look, the more we find that the neuro-muscular associations of “hearing” are part of the great network of associations all across the central nervous system, including the parts of the brain that mediate awareness, attention, memory, and emotion.
Dr. Fan-Gang Zeng (a member of Hyperacusis Research’s Science Advisory Board) wrote about central gain in “Tinnitus and hyperacusis: Central noise, gain and variance.” (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7720792/).
What’s the question? Why is that a question?
Everyone has a list of questions they want answers to. Many organizations publish lists after some process of deliberation that for health-related questions optimally includes researchers, educators, clinicians, administrators, entrepreneurs/business people, and—hopefully—patients and people who care about them. For example, Baguley and Hoare published “Hyperacusis: Major Research questions” in 2018 (https://doi.org/10.1007/s00106-017-0464-3).
Also in the UK, the Hyperacusis Priority Setting Partnership published “Ten Top Hyperacusis Research Priorities in the UK” (https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)32616-3/fulltext), with significant overlap with Baguley and Hoare’s list.
In 2015, Hyperacusis Research convened a “Roadmap to a Cure” workshop to design a useful framework on which to hang researchable questions. https://hyperacusisresearch.org/mission/.
And in a future article, I’ll review a presentation from this year’s ARO by Marlies Knipper called “Too Blind to See the Elephant?” that posed deep questions of tinnitus and hyperacusis that ought to be of wide interest to neuroscientists. If you want to read ahead, her talk was based on a paper of the same name at https://pubmed.ncbi.nlm.nih.gov/34686939/.
These lists have many questions in common, but what’s really important about what they have in common is that answering any of the questions will draw in from many disciplines. One way to get at this essential discipline-bridging is to navigate by questions. We must ask not just “what is the question?” but also “why is that a question?” and “What would knowing the answer do you you/me/us?”
Across the research presented at the ARO meetings, I was guided by questions such as:
- How much of research bore specifically on hyperacusis, versus on the wider biologic context and processes that predispose to, cause, or sustain hyperacusis? (bearing in mind that ‘hyperacusis’ is an experience that not only differs among suffers but has multiple causes such as sudden or repeated noise exposure, drugs, or infections)
- How much does understanding how other auditory disorders work help us understand hyperacusis—and vice-versa? These disorders might include tinnitus, hearing impairment, misophonia, and migraine with phonophobia, for example. But also what are the biological underpinnings of different expressions of hyperacusis, such as annoyance or pain?
- How does research on animal models of hyperacusis or tinnitus inform our understanding of the human conditions? Why would we think it might? With animal models, we can observe aversion or annoyance behavior; that might reassure us that the animals “have” hyperacusis similar enough to the human experience that we can develop treatments, but the way hyperacusis is induced in animal models (usually with relatively brief intense sound exposure or a drug) is different from the way it is in many humans; what does that imply? (More on animal models in the next article)
- Is hyperacusis a ‘final common pathway’ from various types of processes or are there different (though perhaps overlapping) biological processes for the different phenotypes, for example hyperacusis without versus with pain?
- Where is the pain ‘coming from’ with pain hyperacusis? Is that different for different sufferers? Why don’t some people with hyperacusis not get pain?
- Thought experiment: If hyperacusis is a response to damaged OHCs or their synapses on Type II neurons, would perfectly restoring the cochlea in hyperacusis eradicate their hyperacusis? Improving vs. eradicating may depend on whether other structures have sustained permanent damage of dysfunction; and it might differ among individuals.
- To what extent (and how) should patients, caregivers, and clinicians participate in formulating research questions, prioritizing them, and evaluating its value?
Next time:
We’ll focus on animal models and speculate where they might or not yield actionable diagnostic or therapeutic insights on human hyperacusis and tell about some of the amazing presentations at this year’s ARO.

Thank you for all your efforts for us sufferers. I try to donate money again.