Image created with BioRender.com
I come from a musical background and sang as a child. I had a grandmother who struggled with hearing aids when she came to performances. She would get that awful piercing sound to tell her to get her hearing aids charged.
Even as a child, I saw that a lot of therapies for hearing loss were not doing what they should do. They cause more frustration than help. Currently, no therapies are at the point of helping with hearing loss, and they ignore hyperacusis and tinnitus completely.
At the first ARO I went to, I was introduced to Bryan Pollard. It opened my eyes to hear patient stories and to learn what they were experiencing. I heard about setbacks and was thinking about what in our bodies could be causing a setback and how we could research it. It was fascinating to see Bryan’s work ethic and his efforts to link researchers to his cause. He has done an incredible service to this field.
One area that has barely been mined by researchers is social media and hearing what people say in their own words.
Patient stories are very helpful in our trying to understand the human perception of noise-induced pain. It is really important to understand what people are experiencing and to learn what has helped, like protecting their hearing.
Since noise-induced auditory conditions haven’t been helped by mainstream medicine, they have developed their own online communities. People have so many different ways to describe the pathological phenomena, which doesn’t exactly help.
People in these groups may not have the tools to describe what they are experiencing, and that is hampering our understanding of the human experience. But at least now there is some sort of public knowledge that hyperacusis even exists.
The idea of aural fullness, which often accompanies a noise injury, is still a moving target. People describe it differently — fullness, pressure, clogginess.
Within these communities, there is not a unified definition of hyperacusis that encompasses everyone’s experiences. Some of the work Hyperacusis Research has done has been to define the pathology that is affecting humans, which helps give researchers focus in what to look for in creating an animal model.
To move the field of basic hyperacusis research forward, we need to translate the defining characteristics that Hyperacusis Research and researchers within the field have identified, and translate them into testable models.
Several things hamper tinnitus and hyperacusis research. One is not knowing exactly what the causes of these auditory dysfunctions are. We don’t yet have a clear animal model — though that is changing — and there is no way to model an intervention without clinical research.
I have a doctorate in immunology and molecular pathogenesis. I became fascinated with the parts of the body cut off from immediate surveillance, like the eye and inner ear. I am interested in the inflammatory or immune response that happens in the immediate period of time after an injury.
Setbacks are a significant and hidden issue. The idea of setbacks is similar to flares in other inflammatory conditions. With things like multiple sclerosis or rheumatoid arthritis, there are periods of quiescence and of inflammation.
So I want to understand how our inflammatory response to noise could be involved in hyperacusis after a noise injury. The response would involve the immune system cells themselves.
When you have tissue damage anywhere in your body, from a cut on your finger to a sunburn, the cells that are damaged are going to give off signals that create inflammation and pain. I wanted to look at the cell-to-cell communication signals to try to understand how those signals might be related to pain.
One element of pain research is sensitization — a fancy term to say there is increased neural activity when there shouldn’t be. Type II neurons are neurons in the inner ear that are similar to pain neurons in the way they look and the genes they express. If type II neurons act as pain neurons and have increased activity of any type, our hypothesis is that the these neurons may relay a pain signal to the brain.
We have learned that after noise exposure, these neurons would normally have one burst of activity. But if they had previous noise exposure, they would continue to have activity after the initial burst of activity. We call this change in activity a “delayed response.”
When we look at the neuron’s response to tissue damage between the cochlea of a noise-exposed and non-noise-exposed animal, we found this delayed response difference. We do this by poking a tiny hole in the epithelium to cause tissue damage. The type II neurons contain a protein that becomes fluorescent when the neuron is activated.
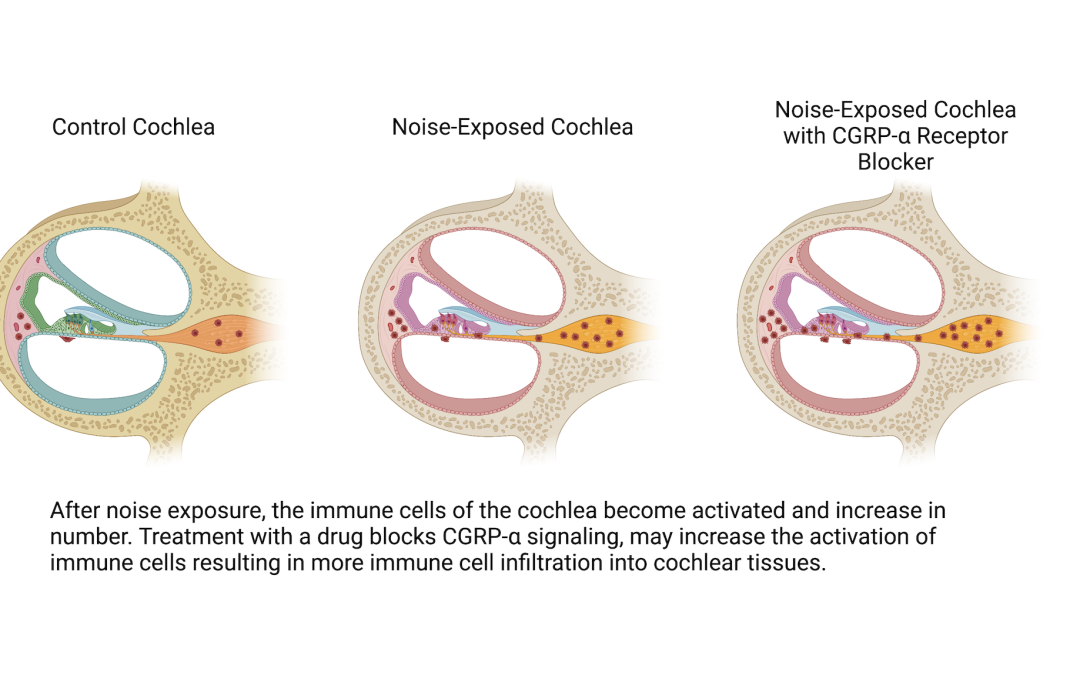
There is a cell-to-cell signal — a molecule called CGRP-α — that is in the inner ear and is also related to pain. My specific project funded by Hyperacusis Research is to look at this signal and see whether that molecule is involved in inflammatory cell migration, which basically looks into whether there is something about inflammation in the inner ear after a noise injury that might be stoking the inflammatory cell response.
We look at mice cochleae a day after noise exposure to see their inflammation status by looking at the number and shape of immune cells, and compare that with mice cochleae that have the CGRP-α cell-to-cell signal blocked by a drug. The data is not yet in, but the result will tell me if signals coming from neurons are involved in inflammation, which may shed light on a neural mechanism for painful hyperacusis.


Thank you for your work. My husband suffers from H & T and just to hear someone spending the time to research this lifts my heart. We are about to move to find a quieter home so he can live in a safe place. He says food dramatically affects his H., especially salt, caffeine, and alcohol, all of which he avoids completely. Of course we try to exist in silence as much as possible. His condition was originally caused by an noise overexposure injury on the job, but now any sound can set him back and some foods set it off as well.
I am now suffering my third bout of hyperacusis and this one is by far the longest. It’s now been almost 2 years and it’s causing me a lot of stress. The one thing I do not suffer the other people seem to is pain. I wonder if that was because I was born with Wardenburg syndrome so I’ve never had perfect hearing. I have found several things which can shut the noise off for a few seconds at a time one of them being to whistle. When I’m doing my knitting I can knit for an hour and not hear anything and I wondered if it was the angle of the head or neck. I feel fairly certain that the damage was caused when my great granddaughter who is profoundly deaf screamed at such a loud volume. I’ve heard recently that there is a possibility that aspirin can cause hearing problems and I have been taking aspirin since having a heart attack nearly 10 years ago. What is your feeling about this?
I have been dealing with painful and loud Hyperacusis with tinnitus for a year now due to sudden loud noise exposure. I’ve had exposure to loud sounds in the past that did not lead to this condition. Is there any research linking Covid shots and boosters to causing this immune cell response?
Great work I developed severe hyperacusis after trauma and sepsis, my cheek can literally twitch when I have hyperacusis pain with noise and vibrations
I suffer from hyperacusis after shunt surgery at John Hopkins in July 2022. I did not have this issue before surgery and have no regrets.
After shunt success for NPH, I am surprised to discover that this condition exist and appalled that it is not temporary, that there is no proven treatment and that I may have to live with this for years. Bummer!!!!
I only only have 21 years to 100 and suffer from the following 4 hearing afflictions
-hyperacusis
-bilateral tinnitus
-bilateral eustachian tube disfunction necessitating annoyingly frequent Valsalva maneuver
-hearing impairment: left ear almost deaf, right ear about 50%
After trying 3 hearing aids and my own NoahLink programming unit I gave hearing aids up. Bone earphones indicated that my problem lies in the cochlea or even further. I dismissed a Cochlea Implant on the bad ear due to uncertainty how my brain would be able to accommodate both the new artificial audio signals from the implant and the accustomed signals from my other ear, also the tinnitus on the (present) better ear would probably remain. My last hope was a treatment by Frequency Therapeutics Inc to regenerate the hair cells in the inner ear, but they gave that up after inconclusive test results. Is anybody else working on regeneration of the hair cells or eustachian tube disfunction?
I suffer with H & T mine has started almost a year ago after having covid. 20 years ago I was diagnosed with menieres which really was difficult to live with but then was offered a trial of steroid injections which were amazing and had no symptoms for 9 years. My life was back to normal, I would say after having covid last December my tinnitus became worse and then started researching what this awful swirling whooshing sound is even when I open my mouth or swallow etc it’s debilitating and I’m now suffering with anxiety and depression which in itself is hard to except as I’m a confident sociable person. I’m searching every day for some hope and help.
I first started suffering after Covid in 2022. After about 12 month it did ease slightly until my Covid jab in October 2023. Now the symptoms have returned – tinnitus, pain with any loud- ish noises, & my own voice echoing in my head. I wear hearing aids but have to turn them down to cope with any noise.
I have prior bilateral ear injury from being exposed to high level noise and vibration work environment for years and I didn’t know what was triggering my migraines, anxiety, sound sensitivity, and tinnitus. I was evaluated by an audiologist that prescribed sound therapy hearing aids to mask my bilateral tinnitus and noise cancellation earplugs to protect my hearing. I have shown some improvement with my right ear. I struggle with random exposures to loud noises that would trigger migraine attacks and sound sensitivity. I find myself having control over my life when I’m home because I can control the noise levels. I had to return back to college to find a career that would accommodate this disability. My audiologist convinced me that I could get through this! As well as my primary dr. She recommended me to become an advocate for this condition.