The Scientific Advisory Board of Hyperacusis Research, Ltd. sponsored a luncheon meeting at the February meeting of the Association for Research in Otolaryngology in Orlando. The goal of the meeting was to promote information exchange about patient experiences of and emerging research in hyperacusis.
This article summarizes the main themes of the meeting, presentations, and attendee discussions. This story was written by Iver Juster, MD, Chair of the Scientific Advisory Board, with review by the presenters.
None of the content in this article should be regarded as providing advice—medical or otherwise.
Panel of Presenters
Steve Barad, MD – Introduction from the Hyperacusis Research Scientific Advisory Board
Jon Wallace – A personal experience with hyperacusis
Dan Polley, PhD – Potential biomarkers for hyperacusis
Megan Wood, PhD – Assays along the neurology of hyperacusis; the role of inflammation
David Eddins, PhD, CCC-A – An in-ear sound management device for loudness-type hyperacusis
Main themes
– Hyperacusis can be profoundly life-changing. It can begin abruptly or be preceded by a period of temporary noise-induced episodes that progress to chronic hyperacusis without or with pain.
– At the most basic level, it’s important to distinguish hyperacusis from other types of sound sensitivity such as migraine or misophonia (a strong aversion to specific sounds such as chewing, rather than sensitivity to sound itself)
– It’s also important to distinguish loudness hyperacusis (strong annoyance that does not involve pain) from pain hyperacusis (“noxacusis”) which involves pain. Also, hyperacusis may occur with or without tinnitus.
– We often focus on noise-induced hyperacusis, but there are other causes or contributors, such as certain drugs, infections, or genetics.
– Noise-induced (and probably some drug-induced) hyperacusis involves both peripheral (the inner and middle ear and auditory nerve) and central (brainstem and above, including sound processing, connections with other areas of the brain having to do with attention, interpretation/ meaning, and emotion).
– Hyperacusis seems to involve dysfunction in information flow to (“afferent”) and from (“efferent”) the central nervous system towards the peripheral auditory components). It is possible that as a person’s hyperacusis evolves, the central mechanisms make long-term structural adaptations. This phenomenon—neuroplasticity—may make it harder to improve hyperacusis over time. To the extent this hypothesis turns out to be true, early diagnosis and treatment could be a key to lessening the impact.
– The audiology (hearing) test called loudness discomfort levels (LDLs) to pure-tone frequencies are part of making the diagnosis and characterizing its severity. For each frequency (pitch), its LDL is established as the quietest tone that just starts to evoke discomfort. But people with hyperacusis often report that complex sounds (e.g., machine noise, crinkling plastic or paper) are distressing at sound levels far below their LDLs. This suggests that auditory processing is involved in producing the hyperacusis experience. Researchers are looking into central nervous system factors that control the response to sound – potentially resulting in treatments that reduce the effect of loud or triggering sounds.
– Work on identifying biomarkers (electrical or chemical signals) for hyperacusis may give us objective tools for diagnosis, management, and treatment studies
– How can we study the biological mechanisms underpinning hyperacusis? We have animal models (including for hyperacusis with pain) but need to better understand how well these models are like human hyperacusis. Also, some tests that can be performed on animals are too loud for people with sound sensitivity. This has implications for testing preventive measures or treatments. It would be helpful to develop assays (ways to measure) deviations from ‘normal function’ at various points along the ascending, descending, and integrative auditory pathways
– Inflammation may play a role in the development and maintenance of hyperacusis, as shown in experiments with the neurotransmitter CGRP (which is involved in both pain and inflammation)
– Stress may be an important factor, for example by influencing central gain or neuroplasticity
– An in-ear programmable device that manages the sound spectrum presented to the ear has shown promise for patients with loudness hyperacusis in a study with about a dozen patients, but the device has not been tested on people with noxacusis. The device also gradually introduced sound therapy as a soft sound thought to reduce the need for central gain. Hopefully clinical trials could be expanded to those with pain hyperacusis to help predict who is likelier to respond to this type of therapy. But many people with pain hyperacusis report they are unable to tolerate sound therapy, perhaps providing an important clue to the biology of hyperacusis.
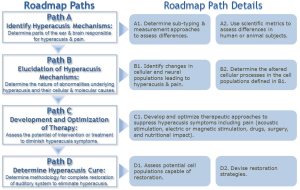
– Moving forward on the Roadmap to a Cure for hyperacusis will involve prioritizing objectives, interdisciplinary planning, consensus definitions of hyperacusis phenotypes, and incorporating researchers, clinicians, patients, bioengineers, and others.
Setting the stage | Pathway to a Cure
Steven Barad, MD kicked off with a welcome, noting Jon Wallace (who has hyperacusis) will describe his H journey; then three researchers will share about their research interests followed by discussion about what we need to do to move forward in our understanding, treatment and helping people live a more normal life. He showed the Roadmap to a Cure framework, emphasizing that we are in early stages of a journey.
Jon Wallace’s hyperacusis story
Jon was living in NYC working as hedge fund manager, dating – and everything was abruptly turned on its head – “it turned on a switch that has never turned off” that included three “strikes” – an airplane trip and exposure to a loud truck followed by fullness in his ears, and then an acoustic shock listening with headphones.
A day or two after the third strike, he says, “my world changed while on a phone call” during which he suffered extreme pain hyperacusis (‘noxacusis’), vibration, and other distressing symptoms. He noticed that ordinary sounds like crinkling of a plastic bottle had become unbearably loud.
Many days he experiences pain as burning from ear to jaw. He also has some light sensitivity. Increasingly, his life is governed by the need to isolate himself to avoid the unexpected sounds that can occur in any situation.
He acquired tinnitus later but noted that for others tinnitus comes first or they arise together. He marked that loudness discomfort levels (a hearing test that checks responses to gradually increasing loudness across a range of pure tone frequencies) don’t paint a valid picture of sound tolerance.
Main themes:
– Progressive through multiple insults (at first temporary, then permanent)
– Ear fullness, pain sometimes radiating to jaw
– Tinnitus
– Sounds can be distorted (crinkling plastic, phone conversation)
– Audiometry LDLs failing to convey an important part of the picture
– He may hear a rumble or warble after exposure to a distorted sound
– Increased sensitivity in other senses – e.g. light
He wondered: was the problem in the middle ear or the inner ear? – (Comment: Does it start there and generalize to the auditory system (and perhaps to the brain? If we have something that can treat it before it generalizes, would that be the easiest and most effective treatment?) This raises the question of how to test what’s happening at each level or interaction or connection along the auditory pathway.
He mentioned a sometimes-performed surgery (cutting the tensor tympani or stapedius muscle that controls how much the middle ear bones that transmit sound energy from eardrum to the inner ear can move in response to a sound) and that hyperacusis was a possible side-effect of the surgery. One meeting participant noted that some people with tinnitus due to myoclonus (sustained involuntary contractions) of the tensor tympani have been treated with muscle sectioning with success. But there may be some hyperacusis sufferers with hyperactive jaw or ear muscles where this therapy would likely not help (comment: and this type of treatment should be regarded as experimental. It may be difficult to figure out whether hyperacusis pain is the result of abnormal muscle tension—or if muscles become tight as a response to hyperacusis due to another cause. There’s evidence for several mechanisms for pain in hyperacusis, which might vary among sufferers).
Dan Polley: Biomarkers for hyperacusis
Biomarker: a defined characteristic that is measured as an indicator of normal biologic processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions. Molecular, histologic (tissue), radiographic, or physiological (such as chemical or electrical) characteristics are types of biomarkers. – FDA.
Dan Polley, PhD (Mass Eye and Ear) (More: https://oto.hms.harvard.edu/people/daniel-polley): Can diagnosis, monitoring and treatment be informed by biological markers of other neurological disorders? Effective treatments are usually identified through large, expensive clinical trials–which are often undertaken only when there is an objective biomarker (e.g., tumor size, seizure, neutralizing antibodies) that can be measured alongside more subjective clinical measures. For this reason, objective biomarkers for hyperacusis are an important – and perhaps necessary – pre-condition for finding an effective treatment.
Having animal models supports the path towards treatments where there are identifiable cellular targets—animals can inform us of what a therapeutic agent might look like. Here again, a biomarker provides the critical ‘Rosetta Stone’ for translating animal research to clinical research. For example, it is very difficult – perhaps impossible – to claim that an animal can model the complex neurological and psychological presentation of hyperacusis symptoms. But it is entirely possible to show that an objective biomarker measured in humans with hyperacusis can also be identified in animals. And with this Rosetta Stone identified, the power of animal models to test therapies at a cellular and molecular scale in controlled laboratory settings can be realized.
Next, what biomarkers should we seek for monitoring hyperacusis severity or to measure the response to treatment? As we seek candidates for such biomarkers, we need a ‘gold standard’ which we agree that by definition always correctly identifies hyperacusis and distinguishes it from non-hyperacusis (including other types of sound sensitivity); then we compare a candidate test—such as a biomarker—to the gold standard. Currently, we think of questionnaires (such as the Khalfa’s Hyperacusis Questionnaire (see endnote 1)) and the loudness discomfort level (LDL) test.
Comment: Beyond being acceptable to people with a high degree of sound sensitivity, the test would need to be able to distinguish hyperacusis from other kinds of sound sensitivity such as the phonophobia of migraine, and from misophonia (a strong aversion to specific sounds such as chewing). Optimally we could discover biomarkers to differentiate pain hyperacusis (noxacusis) from loudness (but without pain) hyperacusis.
Key point: Biomarkers would be useful in gaining insight into the biology of hyperacusis in its different expressions and could point to highly personalized treatments or preventive measures.
To accomplish this, we’d need to establish a promising connection between potential biomarkers of hyperacusis and the ‘gold standard’ tests to attract a company that can spend many millions of dollars on clinical trials. To explore potential biomarkers, Dr. Polley’s lab has been working on ‘signatures’ – behavioral clues, such as facial expressions, that researchers have used in animal models; and specifically on signatures in the pupils of the eyes. And they can drill down to the cell types that are markers. They exhibited 2 posters (see endnotes 2 and 3) at ARO showing cellular markers of hypersensitivity to sound after noise – they’ve found a target in the auditory cortex, in GABAergic neurons (see endnote 4).
Activating these target neurons can downshift a mouse’s loudness perception by 15 decibels (dB)—enough to make a significant difference for someone with hyperacusis (see endnote 5). (Comment: In this case, ‘activation’ produces neurological inhibition, not excitation). This type of cell becomes hypoactive after noise-induced hearing loss. This creates a potential path toward treatment, by reinvigorating the inhibition this cell normally provides, laying out a possible route towards shifting sound sensitivity down towards the normal. Their ambition is to begin clinical trials in the next few years.
If it can be shown to correlate with improvement in hyperacusis, reinvigorating the inhibition might function as an “intermediate” outcome measure (Comment: like blood pressure is an intermediate outcome for its associated ultimate outcome, stroke). His lab is fortunate to operate where there are human and animal research labs that can collaborate or communicate their findings and ideas—generating crosstalk between animal models and human systems.
Meeting participants’ discussion
Question: To what extent do animal models (which are usually based on inducing hyperacusis with noise) relate to human hyperacusis? Not all people get hyperacusis after noise exposure. Others get tinnitus or hearing loss, or none of these…whereas with mice nearly all get noise induced injury: the lab mice are inbred so have very similar genomes and phenomes (observable characteristics). The trigger for hyperacusis isn’t just what happens in the ear – it’s in the brain, so if we could measure what directly controls the brain response we could accelerate research on what modulates hyperacusis. But currently, we can’t measure the thing that’s directly responsible for that.
Question: What about situations with no known noise exposure, yet hyperacusis develops? (Comment: this idea was presented at Dr. Ben Auerbach’s Hyperacusis Symposium talk).
Are there similarities between noise sensitivity such as might occur with or between migraines and hyperacusis? Does this shed light on the role and influence of central sensitization?
Also aging might interact with how the auditory system responds to noise.
Megan Wood: Assays along the neurology of hyperacusis; inflammation
Megan Wood, PhD (Johns Hopkins) (More: https://hearinghealthfoundation.org/meet-the-researcher/beers-wood2022).
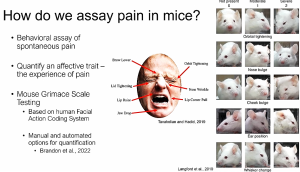
Dr. Wood’s lab is working to understand mechanism behind pain hyperacusis (noxacusis) by developing an assay for pain perception in a rodent model – based on a facial action-response coding system. Researchers often use a facial grimace test to indicate a heightened response to sound in mice, but even in a genetically homogeneous mouse model not every mouse develops hyperacusis. This suggests that susceptibility varies even among genetically-identical mice.
Facial grimace in response to pain is evolutionarily conserved, and humans’ response is similar to that of rodents. This assay is also used in other nonverbal animals, including newborn humans. It includes different types of facial movements and changes in the position of the outer ear pinna. The coding system was painstakingly developed by making judgment calls looking at videos frame by frame, but recently they have used machine learning to improve automated detection of signals. Besides saving time and standardizing scoring and interpreting responses, machine learning also aims to remove bias.
Here is a slide from her talk at the ARO Hyperacusis Symposium, that shows similarities between human and mouse facial grimacing in response to pain.
To move forward with hyperacusis, we need to come up with a framework where we have a series of assays that can be compared when doing different types of intervention.
We’d first establish what happens to a rodent after exposure to a single loud sound, starting with auditory brainstem responses (mapping passage of information along the first part of the central auditory pathway), and correlate that with what changes about their hearing and in the inner ear.
This framework offers the ability to use in models with different gene variants, or to introduce a drug before or after administering the sound. Dr. Wood was struck by the episodic nature (variable as well as constant symptoms) in some patients’ descriptions – reminiscent of inflammatory disorders. Her work with immunity led to looking at calcitonin gene-related peptide (CGRP) , which comes from auditory neurons. We know that CGRP modulates pain perception in the skin and modulates activation of immune cells. It may not be only CGRP, but with this framework we can start to pick apart the different pathways that contribute to hyperacusis in mouse models, even comparing mouse models that have different inflammatory responses to any sort of insult like noise. Different mice strains differ in their immune responses to stimuli.
Importantly, the auditory pathway has highly complex interactions with all of the cranial nerves, thus influencing and modulating how auditory information coming from the cochlea is processed, experienced, and interpreted. Acoustic stimulation can cause inflammatory cell infiltration even in the brainstem, and also in the inner ear (as well as in the cochlea). Central nervous system (CNS) control via fibres descending from the CNS to the neurons of the auditory system is potentially a major component – there are more pathways coming down from the brain than into the brain.
Comment: We need to think across research and clinical disciplines.
You can read about Dr. Wood’s research here and here.
Dr. Wood presented an update to her research on pain hyperacusis at the Management of the Tinnitus and Hyperacusis Patient conference at the University of Iowa on August 10.
David Eddins: An in-ear sound management device
David Eddins, PhD, CCC-A (University of South Florida) (M
ore: https://www.usf.edu/cbcs/csd/faculty-staff/eddins-david.aspx) – Audiologist.
Dr. Eddins discussed his research with Craig Formby, stemming from data on the effectiveness of sound therapy in patients with tinnitus, hyperacusis, and both. They observed that patients using managed sound therapy for patients with tinnitus—but who had some degree of hyperacusis—experienced gradually rising loudness discomfort levels, and so postulated that sound therapy might be helpful for at least some types of hyperacusis (such as loudness but without pain).
He noted that there’s also converging evidence from various studies on sound therapy—for example around sound deprivation and augmented acoustic environments—that point to plasticity (the biological ability to adapt to circumstances) in the central auditory system based on lack of or supplemental sound exposure. For example, in one experiment, college students (who did not have hyperacusis) who wore bilateral earplugs 23 hours a day had lowered loudness discomfort levels (one measure of sound sensitivity) after two weeks.
This finding suggested to the researchers that the central nervous system tries to compensate for impaired peripheral stimulation. Similarly, wearing low-level sound generators (“sounding like listening to a seashell”) 23 hours per day for 2 weeks was followed by increased LDLs.
Hypothesizing that blocking sound as a coping mechanism in hyperacusis might exacerbate the condition by training the central nervous system to ‘turn up the volume,’ they studied patients with loudness (not pain) hyperacusis (so they don’t know to what degree their process and outcomes would translate to pain hyperacusis).
To reduce anxiety about taking away ear protection, the device included an adaptive intervention with electronics to cut off exposure to sounds above a loudness threshold. The device is an ear mold that expands at body temperature, for a precision fit. The ear plug is connected via a thin hollow tube to an ear level device. When the wearer has accustomed to the sound-protection part of the device, they then add in the low-level seashell-like sound.
The device aims to amplify sounds enough to get to exactly the gain needed to overcome the blocking of the ear plug (“unity gain”). This gives the device 20-40 decibels (dB) of headroom for locking out high level sounds. They use amplitude compression, which is the output-limiting feature of a hearing aid, adjusted to the wearer’s LDL not to pure tones but to speech.
Thus the device provides protection to high level sounds but maintains exposure to comfortable sounds. Over time, as the sound therapy gradually expands the dynamic range, the device is tuned to give the patient increasing exposure to environmental sounds.
Hyperacusis Research founder Bryan Pollard helped them develop the project. They built the device in their lab and tried it on 12 subjects (ages 20s to 60s) with hyperacusis (but not pain) for six months. Half of the subjects also had tinnitus (which was not assessed as an outcome). LDLs at the end of 6 months had increased on average by 35 dB. They measured LDLs monthly and found they could gradually back off on output-limiting. LDLs continued to improve at 6 months (when the study ended), as did scores on a hyperacusis questionnaire, and some of the subjects said that their ability to tolerate sounds that were previously intolerable had improved.
In the next phase they want to include more objective physiological measures. The investigators plan to submit an NIH proposal for a larger group but as yet have no funding from industry, including hearing aid manufacturers who would want to see its potential as a profitable investment.
Meeting participant discussion
We don’t know to what extent the hyperacusis phenotypes (loudness, annoyance, fear, pain ) relate biologically—we need to come to consensus with standard nomenclature that better recognizes what might turn into better research on genomics, biochemistry, neurology. This will accelerate developing evidence based tools. Distinguishing loudness from pain hyperacusis is a step forward.
(Comment: I wonder whether the often-observed trajectory where a person starts out with loudness hyperacusis, then proceeds to with-pain, is a third phenotype or represents a biology that is actually common to both phenotypes?) Perhaps this is a parallel to multiple sclerosis where two phenotypes were once considered different but now look like they might be expressions of the same underlying biology at different stages?)
Dr. Richard Tyler mentioned evidence that supports stress as an important factor. In one experiment, they gave mice stress hormones for 2 months and found decreased sound tolerance. So it seems important to also consider one’s background emotional state – we might be more susceptible to being bothered at some points. “Our Psychological Model of Hyperacusis separates the perception of loudness (or pain) from the reactions to the loudness (or pain).” However, the stress-susceptibility model may not be universal.
Finding animal models that allows us to break apart these different aspects will be really important. We need measures of the percept (the person’s experienced perception) itself and the reaction to the percept.
It will be helpful to understand the biology behind why complex sounds (like power tools, crinkling paper) much quieter than one’s pure tone LDLs can be so bothersome.
Hyperacusis Roadmap to a Cure
Notes
- https://tinnitustherapy.org.uk/hyperacusis-questionnaire-hq/.
- Cortical Parvalbumin GABA Neurons Regulate the Perceptual Volume Knob: Bi-Directional Changes in Loudness Perception via Reduced or Enhanced PV-Mediated Inhibition in the Auditory Cortex
- Central Gain is Significantly but Equivalently Elevated in Hidden Hearing Loss, Tinnitus, and Hyperacusis, Suggesting an Upstream Pathophysiology Unrelated to Any Particular Perceptual Phenotype
- A GABAergic neuron is a neuron that recognizes signals from other neurons that are carried by the neurotransmitter molecule GABA (gamma-amino butyric acid), which is the primary inhibitory neurotransmitter in the brain.
- It should be noted that sound intensity as measured by dB is only one component of sound tolerance for people with hyperacusis. Others may include the overall sound quality (for example, paper rustling or a leaf blower a block away may have low sound intensity but still cause pain), time of day (neurological fatiguing), etc.
- https://en.wikipedia.org/wiki/Calcitonin_gene-related_peptide
- One subject didn’t get measurable benefit, but that subject had several complications and wasn’t fully compliant with wearing the device but remained in the study.
- https://hearinghealthfoundation.org/types-of-hyperacusis







This can get so bad it forces you to kill yourself. It needs to be medically noted that this can be. It’s not fair to people who die because of reactive tinnitus and pain hyperacusis to shove it off on mental when they have been locked indoors and poisoned and tinnitus and hyperacusis have no limit to how bad they can be. Thank you all for pushing toward a treatment or a cure or more knowledge. Auditory specialists across the world need to be in touch with y’all and relearn everything they know about tinnitus and hyperacusis so they stop sending people to their death with ototoxic medications and telling them to not over protect and cause them more damage…when silence and no meds is best for these conditions for most patients. They don’t tell someone with a broken leg to walk on it. Why do they do that with someone that has damaged ears and are sensitive to sound and unstable tinnitus. So wrong.
Thank you all again. Much love and God speed.
I don’t understand the comment of T ‘silence and no meds is best’. I read the summary of this symposium and read that early diagnose and treatment is important because we adapt so quickly. So if you avoid noise, you may tolerate noise less. If you read about the study where they have soft noises for two weeks for people without pain version of hyperacusis, it makes them more tolerating to louder noises.
I wonder if there is consenses about not to seek silence (the first reaction if you experience pain hyperacusis). Cause now you may know in early stage to have pain hyperacusis, but it’s unclear how to deal with it in the best way possible.. and in the symposium it opens that you might have to act fast or treatment will not work as good later on.
I am a DJ and after more than 10 years of DJing one day in 2014 I woke up with extreme sensitivity to high frequencies. It could be the sound of dishes clinging together or as subtle as water pouring onto a surface. Super sensitivity basically to all sounds but infparticular high frequencies. Then I went to find a doctor, normal doctor told me to go to an ear doctor, I went to one, they did some scans, sent me to another doctor, the final doctor I went to basically told me I was completely imagining it, and it’s a mental issue. I was like WHAAAA, I was in a heavy metal band when I was younger, and DJ’d for 17 years up to that point, it made sense why my ears would have some damage only to have some doctor, tell me that the physical pain in my ears is all just my imagination. So I had to figure it out myself. I did hear that white noise helps, and I tried that, and also that even just exposing yourself to atmospheric noises (could be car traffic, beach waves, anything that is not too close or harsh) does seem to be theraputic, but ultimately another 10 years has passed and I still have the problem. It’s much better than that first day I woke up with pain, but now I always have to wear ear plugs in bars and clubs or my ears will overdrive and I can hear crackles and crumbles if noises are too loud, or if say I am in a bar and a male has a very basey voice and they are shouting to be heard over the music, this really hurts my ears. Even the bass in my own voice hurts my ears a little. So that’s my story, would love to see a cure one day and not be told I am imagining a life debilitating problem.
I have been recently diagnosed with hyperacusis in 2020, but have had the symptoms my entire life. When I was a kid, I was able to hear a CRT television be able to turn on or off with no volume. Noone knew what was going on. Whenever someone got in an angry or vehement situation, I just went crazy. My ears have always hurt. I endured all of my childhood with extreme irritability to the most common sounds. I tell you, I think mechanical sounds are the worst. Fast forward to 2021, I got my first pair of hearing aids designed for hyperacusis, and I had to take them back because they weren’t covered by insurance. Then my insurance was able in 2022 was able to cover them and I saw another specialist I really had many problems with even the hearing aids, as I put them on, I see little difference about half the time, even though I like some of the features, the only thing that really helps is positivism and non-discrimitatory sounds. Prays going forward as hyperacusis is as bad as they say.